Research Highlights for the Curious!
A series of grant-funded studies in the past 5-6 years in my labs in Germany, USA and Chile, with collaborators and my former doctoral student Dr. Josue Dalboni da Rocha (now a postdoc in Geneva), has resulted in the development of scientific and computational methods for the investigation and diagnosis of Alzheimer’s disease using cross-disciplinary approaches of neuroscience, brain imaging and Artificial Intelligence (AI). This work has resulted in 3 peer-reviewed international publications and a patent (references to original publications are given at the end of this article).
Our work is in the spirit of research conducted worldwide by many labs and research institutions to understand this complex brain disorder and to develop accurate methods of diagnosis and prognosis (i.e., prediction of treatment and disease outcome in individual patients).
Alzheimer’s disease (AD) is a brain disorder that results in decline of memory, thinking, cognitive and emotional abilities, and disrupts the patient’s ability to live independently. AD is a type of dementia caused by the progressive decline of brain cells with age. The disease was named after the German psychiatrist and neuropathologist Aloysius Alzheimer who identified the first case of what was called presenile dementia, now renamed Alzheimer´s disease. Coincidentally, Aloysius Alzheimer studied medicine during 1886/87 in the University of Tuebingen in Germany, the same university where I completed my PhD in Neuroscience.
An early sign of the disease is memory loss that results in difficulty in remembering things and managing events of daily activity, which could be noticed both by the individual as well as family members. As the person ages and the disease progresses, memory impairments worsen; e.g., forgetting conversations and names of family members, getting lost in previously familiar places, routinely misplacing objects, and so on. Other cognitive problems, related to thinking and reasoning, making decisions, planning and conducting daily chores, and changes in personality and behavior, such as depression, mood swings, social withdrawal, changes in sleeping habits, loss of inhibition, etc., develop over time.
Why and how is the disease caused? Does science know enough about it? Is there accurate diagnosis and treatment for the disease? To answer the above questions, we need to understand the physiology of the brain.
Neurons or nerve cells are the computational units of the brain. By one estimate, the human brain has about 100 billion neurons. Each neuron is connected to 10,000 other neurons through synapses which are microscopic gaps in which electrochemical signals are passed. Each neuron contains a cell body (also called soma), several dendrites (branching filaments attached to the cell body) for receiving signals from other neurons, and a single, long filament called axon that carries signals from the soma to other neurons.
How do neurons function? The neuron´s cell body, i.e., soma, maintains a certain level of electrical voltage across its cell membrane, between the inside and outside of the cell. When the neuron receives electrical signals from neighboring neurons, its voltage changes, and if the change in voltage crosses a threshold, the neuron is said to “fire”. When the neuron fires, it sends current, also called the action potential, along its axon to be received by other neurons connected to it. The electrical signal passes swiftly through the axons and is received at other neurons at the featherlike dendrites and are passed across to the receiving soma via synaptic gaps by complex electrochemical messaging. Neurons receive, process and resend electrochemical signals in a network of connections with other neurons via junctions called synapses.
Neurons thus connect with other neurons in complex cell assemblies called neural networks. Although neuroscience research has helped us understand some aspects of the functioning of the brain in producing sensation, perception and action, much more remains shrouded in mystery and misunderstanding, leading some scientists to claim that understanding the human brain, especially related to the complex cognitive processes such as consciousness, is the last frontier in scientific discovery. Personally, these challenges along with the interdisciplinary nature of the field has attracted me to neuroscience research. The challenges of understanding the brain processes underlying healthy mental life while identifying and overcoming its abnormalities such as in Alzheimer’s disease, remains to be a tall order.
Coming to the questions of Alzheimer´s disease, there is no cure for the disorder so far, although there are medications that relieve symptoms, slow the rate of progression of the deterioration and help in achieving limited level of independence by the individual for some time. For these medications to be effective, early diagnosis of AD is critical. However, early diagnosis disease could be adversely affected by the fact that the symptoms are often confused with those of Mild Cognitive Impairment (MCI), a transitional condition that does not always develop into AD.
Past research has identified physiological and biochemical changes in the brain in Alzheimer’s patients. These underlying causes of the disease, called biomarkers, include toxic protein deposits outside neurons (i.e., extracellular deposits) called amyloid-ß proteins, tangles of fibrous elements called tau proteins and certain types of plaques. A liquid that cushions the brain and helps in other functions called the Cerebrospinal Fluid may show signs of these proteins, but this test is highly invasive and cannot be conducted without surgically operating the brain. Blood tests can also show these harmful proteins and other molecules called lipids within 2-3 years after the onset of the disease. However, non-invasive methods for much earlier detection of the chances that a person may get Alzheimer’s disease are urgently needed to helpful in distinguishing AD from less harmful MCI, and for the application of targeted treatment to slowdown neuronal degeneration and provide support to the patient.
To this end, many labs in the world are applying a variety of techniques in biomedical sciences, including Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI) that measure brain’s structure, function and chemical composition at high resolution.
In my research with Josue and collaborators, we used a sophisticated technique of MRI called Diffusion Tensor Imaging (DTI). Using this technique, we acquired high resolution images from AD and MCI patients. With sophisticated image processing, we extracted from these MRI images information about the white matter fibers that connect groups of neurons in different parts of the brain.
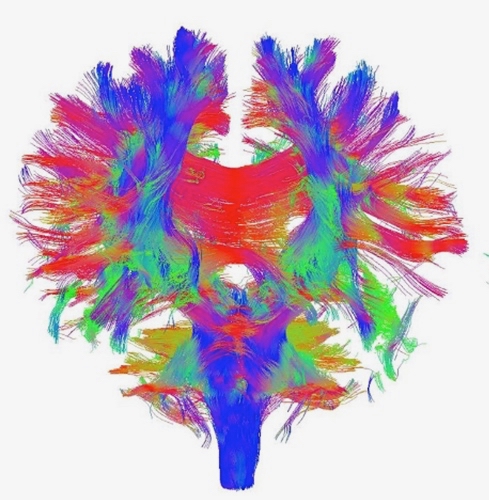
DTI works by measuring the diffusion of water molecules inside millimeter-sized three-dimensional pixels, called voxels, of the MRI images of the brain. Water diffusion within the axons follow specific directional pathways in contrast to omni-directional water diffusion in the brain tissue outside the axons in the brain tissue. Using sophisticated tensor mathematics, we can estimate the direction of the diffusion of water to graphically represent the axonal fibers that connect different regions of the brain (see Figure 1). The rapid developments in this sophisticated technique, called Fiber Tractography, has enabled a slew of investigations of the human and animal brains in a non-invasive manner (without surgically operating the brain).
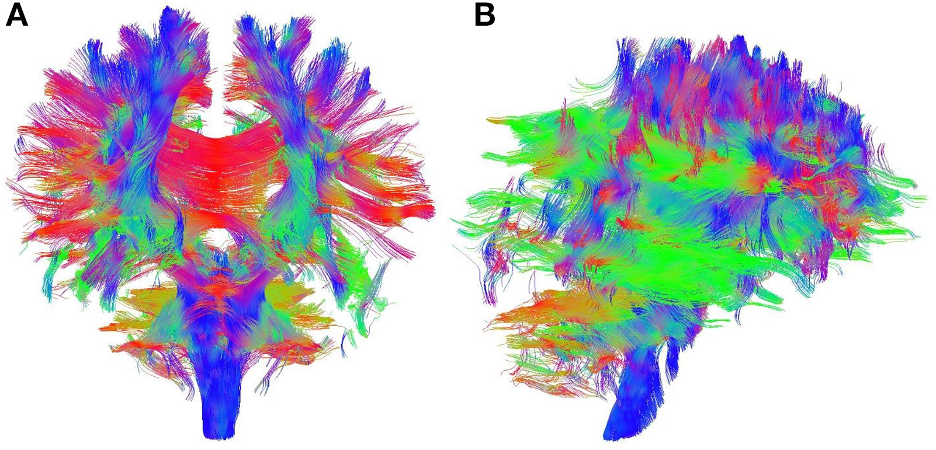
Figure 1. The brain´s intricate axonal connections obtained from Diffusion Tensor Imaging, A – front view, B – side view. Blue lines show connections in the superior (top) – inferior (bottom) direction, green lines in the anterior (front) – posterior (back) direction, and red lines in the medial (inner) – lateral (outer) direction (adopted from Ebadi et al 2017).
Josue and I extended the above technique to develop a mathematical representation of axonal fiber connections of the patients´ brains from the MRI images, using principles of a type of applied mathematics called Graph Theory. Once the brains of the patients were represented as mathematical graphs (see Figure 2), we could analyze the graphs using analytical methods of graph theory. We could extract properties of the graphs, in terms of how strongly and densely the different parts of the graphs are connected using software programs. Our idea was that such properties could be different between AD and MCI patients, based on the expectation that their brains could be wired differently. In other words, we hypothesized that the AD brain is abnormally connected in different parts than the healthy human brain or the MCI brain. To test this hypothesis, we submitted the extracted properties of patient graphs to an AI algorithm that could learn to recognize the differences in the network patterns of the AD and MCI patients.
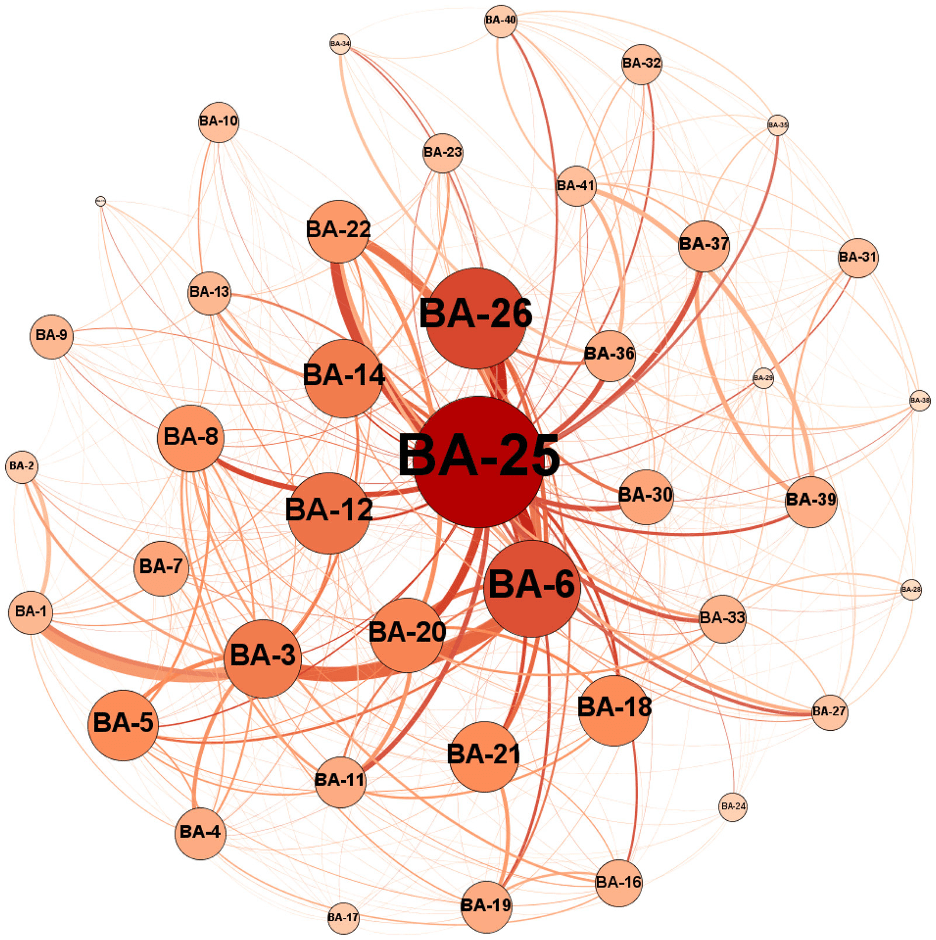
Figure 2. An example of a graph representation of the brain´s connection of a patient. The circles represent different brain regions (groups of neurons) with their scientific names inside the circles and the curved lines represent the axonal connections between the brain regions. The diameter of circles are proportional to the number of axons connecting the regions, and the thickness of the curved lines indicate how strong the connections are (adopted from Ebadi et al 2017).
Our results were quite successful showing that the AI algorithm could distinguish between AD and MCI conditions in new patients with 93% accuracy. We also found out that a region of the brain called Hippocampal Cingulum (see Figure 3), involved in memory formation and retrieval, was a key region in AD. With further advances, we hope that our technique can be used by clinicians for more accurate diagnosis and targeted treatment of AD and MCI in the future.

Figure 3. The blue area shown on the two left (A) and right (B) hemispheres of the brain is called the parahippocampal cingulum. This area is found to be affected in Alzheimer´s disease in comparison to normal individuals and Mild Cognitive Impairment (adapted from Dalboni et al 2020).
Our technology has now been developed into a generalized approach for pattern recognition of brain connections in neurological patients. We have now patented the technology, and is applying it to other brain disorders, such as Multiple Sclerosis with neurologists in Chile as well as in Columbia University, New York.
Despite this progress in our research, the challenges of diagnosis and treatment of AD and other dementia remain to be overcome. Much work still needs to be done, but the excitement and fulfilment of passing a minor milestone would keep us encouraged to continue our research. For detailed scientific and technical information about the work, please see the references for our peer-reviewed journal papers and the patent below. You can download the original journal articles by clicking on the download links below.
References
- Dalboni da Rocha JL, Bramati I, Coutinho G, Tovar Moll F, Sitaram R. Fractional Anisotropy changes in Parahippocampal Cingulum due to Alzheimer’s Disease. Sci Rep. 2020 Feb 14;10(1):2660. doi: 10.1038/s41598-020-59327-2. PMID: 32060334; PMCID: PMC7021702.
- Dalboni da Rocha JL, Coutinho G, Bramati I, Moll FT, Sitaram R. Multilevel diffusion tensor imaging classification technique for characterizing neurobehavioral disorders. Brain Imaging Behav. 2018 Dec 5. doi: 10.1007/s11682-018-0002-2.
- Ebadi A, Dalboni da Rocha JL, Nagaraju DB, Tovar-Moll F, Bramati I, Coutinho G, Sitaram R, Rashidi P. Ensemble Classification of Alzheimer’s Disease and Mild Cognitive Impairment Based on Complex Graph Measures from Diffusion Tensor Images. Front Neurosci. 2017 Feb 28;11:56. doi: 10.3389/fnins.2017.00056.
- Zurita M, Montalba C, Labbé T, Cruz JP, da Rocha JD, Tejos C, Ciampi E, Cárcamo C, Sitaram R, Uribe S. Characterization of relapsing-remitting multiple sclerosis patients using support vector machine classifications of functional and diffusion MRI data. NeuroImage: Clinical, September 2018.
- Patent: System and method for clinical diagnosis of Alzheimer’s disease and Mild Cognitive Impairment using multilevel pattern classification of MRI Diffusion Tensor Images. Inventor: Ranganatha Sitaram. Filed December 2017.